Research
Research in our lab focuses on the development of tools for single molecule based super resolution microscopy, protein trafficking and sub-cellular physiology.
Protein Trafficking
Tools for protein trafficking: The endocytic/exocytic processes are fundamental to a wide range of biological phenomena, including immunity, allergy and synaptic transmission. Utilizing engineered proteins that fold well in the secretory pathway of cells and bind to otherwise weakly fluorescent dye molecules, we have developed a series of fluorescent pH indicators that are “activated by targeting.” These ratiometric dyes enable direct interrogation of the endocytic trafficking process and the protein fate after stimulation by biological ligands or drugs. Trafficking of receptors and transporters under the influence of genetic mutations and pharmacologic treatments provides new mechanistic and therapeutic insights into these important biological processes.
Single Molecule Imaging
Tools for single molecule imaging: The brightness and photostability of quantum dot materials have made them attractive fluorescent labels for single molecule imaging, yet the size and ease of conjugation to biological targets are still practical challenges for most experiments. We are developing novel genetically encoded proteins, called fluorogen activating peptides (FAPs) that bind and activate quenched light-harvesting polymer materials made from multiple chromophores (fluorogenic dyedrons). This technology allows bright, stoichiometric labeling using a genetically fused expression tag, and can be applied to both purified protein labeling for biophysical measurements and to measurements in living cells.
The noncovalent nature of the FAP-fluorogen system gives rise to interesting photostability properties that can potentially be exploited in single molecule imaging. We are investigating and optimizing the photostability of FAP-fluorogen complexes with the goal of producing compact, bright, photstable genetically encoded tagging strategies as an alternative to quantum dot labeling.
Sub-cellular Physiology
Tools for sub-cellular physiology: The binding of a fluorogen to a fluorogen activating peptide can be used to activate fluorescence of multichromophore structures. Exploiting efficient intramolecular energy transfer from an environmentally sensitive donor, we are developing a family of ratiometric indicators that are targeted to a genetically encoded peptide and ‘activated by targeting’ to a fluorescent state. Different designs of these probes result in either dual-band excitation or dual-band emission, which can be used to build quantitative indicators. Sensors for a range of analytes are possible, but immediate targets include mitochondrial potential, peroxisomal stress, reactive oxygen species, and biological cations.
Superresolution Imaging
Tools for superresolution imaging: Genetically targeted probes are very useful for imaging dynamic changes in living cells. Recently, it has been possible to image beyond the conventional diffraction limit of light by exploiting transitions between states in fluorescent molecules. Using fluorogen activating peptides, we have shown that STimulated Emission Depletion (STED) microscopy is practical in living cells, achieving a 2-3 fold enhancement over conventional microscopy. Using the equilibrium binding and activation of fluorogens by their cognate fluorogen activating peptides, we are developing new approaches for sparse labeling useful in single particle tracking and localization microscopy.
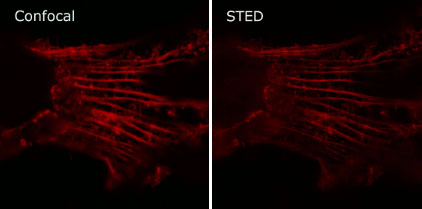
Confocal (Left) and STED (Right) images of actin filaments labeled with FAPs
HTS of Nanobody Affinity Reagents
Secrete, Capture, Sort, Sequence: NGS Decoded Molecular Recognition Pairs: Despite the predominance of monoclonal antibody based technologies, the means by which monoclonal antibodies are generated and disseminated, together with the size and structure of IgGs, impose fundamental limitations on their usefulness, especially for research purposes. Poor specificity and documentation has led to a crisis in experimental reproducibility, leading to an annual waste of ~$350 million in US research expenditures. Rectifying this by the systematic generation of sequenced, validated monoclonals would cost an estimated $50,000 per antibody and would fail for many targets.
Limitations of monoclonals will be addressed by developing a yeast ‘secrete and capture’ co-display system for the high throughput isolation and improvement of recombinant nonimmune Nanobodies (NBs), small protein affinity reagents derived from camelid antibody VHH domains. Fluorogen-based FACS technology that quantifies displayed NBs and captured target protein domains (TPDs) will be integrated with next generation sequencing (NGS) that identifies the associated complex. Integration will greatly expand the repertoire of biological targets for which cognate NBs may be isolated, and facilitate the creation of focused NB toolkits.
Current nonimmune scaffold screens use purified target protein to isolate candidate binders that are physically cloned and individually evaluated. This resource-intensive approach will be replaced by the following:
(1) A FACS reporter assay that quantitatively reports the interaction of co-expressed NB and TPDs in terms of specificity, affinity and kinetics, thus avoiding the use of purified protein. The assay is based on fusing surface-displayed NBs and secreted TPDs to spectrally distinct fluorogen activating proteins (FAPs) that fluoresce when binding non-fluorescent dyes (fluorogens); fluorogens may flexibly linked by PEG as a ‘tie-dye’. A cleavable tie-dye is used to stabilize and report on a cell surface complex of secreted TPD and cognate displayed NB; upon cleavage, kinetic analysis of TPD-FAP release from the cell surface allows one to estimate NB/TPD affinity.
(2) A method that physically fuses the encoded genotypes of co-expressed NB and TPD, enabling NGS analysis to resolve FACS assays of complex populations into the binding phenotypes of individual clones, thus eliminating the need for physical cloning. After mass mating of yeast libraries respectively encoding NBs and secreted TPDs on separate plasmids, CRISPR will be used to force bulk recombination of sequences encoding the NB and a barcode that identifies the associated TPD into a single NGS decodable read frame.
(3) Multiplexed screens in the form of bioinformatics derived TPD query sets to directly obtain groups of related reagents, thus minimizing the need to serially evaluate individual clones. Queries will be used to isolate NBs that probe biological functionalities that were previously very difficult to approach; our test cases will be: (i) neuroligin splice isoforms; (ii) neuroligin/neurexin complexes; and (iii) bacterial surface protein ectodomains.
NIH/NIGMS Grant 5R01GM126657