Department of Biological Sciences at Carnegie Mellon University
Research
The APC Tumor Suppressor and the Cytoskeleton
Cancer is the “Emperor of All Maladies”1. This collection of related, but molecularly distinct diseases, will affect almost 40% of people in their lifetimes2. Through decades of research scientists have made tremendous strides in preventing, treating and curing specific types of cancer. However, the full scope of cellular dysfunction that contributes to cancer is still not known. To answer this question, we must first develop a comprehensive understanding of how normal cells work.
Cells migrate, divide, and change shape in extraordinary ways to produce tissues, organs, and organisms. Mutations in the genes and changes to the proteins underlying these basic cell functions can drive the initiation and progression of diseases like cancer. Behind the scenes of all of these remarkable cellular acrobatics is filamentous actin, a deceptively simple polymer that can be assembled, bundled, branched, cross linked, severed, and disassembled by a cadre of actin associated proteins to produce the amazing variety of actin based structures animal cells need. How cells are able to construct distinct actin filament arrays of different sizes, architectures, and dynamics from a common pool of building blocks is largely a mystery.
Our lab uses the fruit fly, Drosophila melanogaster, as an experimental model to discover the molecules and mechanisms that govern the assembly and function of specific actin structures in cells during development. Some of the many different populations of actin that we study in the lab play critical roles in the process of oogenesis. Specifically, we are using the striking arrays of colossal actin cables (~30 µm long, ~25 filaments thick) that develop in nurse cells late in Drosophila oogenesis as a model to study how two families of proteins, APCs and formins, control the formation of new actin structures. Adenomatous polyposis coli (APC) is a human tumor suppressor responsible for initiating roughly 80% of all colorectal cancers with roles in Wnt signaling and cytoskeletal function, and formins are a diverse family of actin assembly factors.
Together, these proteins collaborate in unique ways to regulate the development of the actin cable arrays in oogenesis. We are coupling our expertise in Drosophila genetics and cytoskeletal cell biology with powerful in vitro reconstitution assays and single molecule Total Internal Reflection Fluorescence (TIRF) microscopy provided by the lab of our collaborator Bruce Goode at Brandeis University. Together, we are gaining significant insights into the detailed molecular mechanisms, cellular functions, and development consequences of APC-formin partnerships that are contributing to our understanding of APC’s role in human tumor suppression.
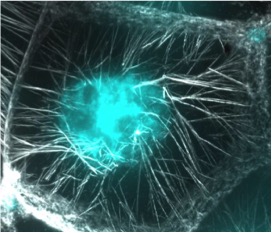
Figure 1: This is one nurse cell in the late stage Drosophila ovary. The actin cables stained with phalloidin (gray) are elongating toward the nucleus (cyan) where they make contact and push the nucleus toward the opposite end of the cell. This enables the nurse cells to successfully dump all of their cytoplasmic contents into the oocyte without the nucleus getting in the way. This is a critical step in the successful production of a mature egg ready for fertilization. APC proteins and formins are required for the normal assembly of this actin cable array.
Notes:
1 ”The Emperor of all Maladies: a biography of cancer” by Siddhartha Mukherjee, M.D. (2010) and a documentary by Ken Burns (2015) based on the book
2 Statistics from the National Cancer Institute based on 2010-2012 data
“Mind-Altering Bugs”
We are all too familiar with humanity’s “modern plagues”3, diseases and disorders that appear to be on the rise particularly in industrialized countries. These may include obesity, diabetes, allergies, autism, and mental illness. These take a tremendous toll on individuals, families, society at large and the economy. What has changed in our world and in our biology to drive the high rates of these problems? We are beginning to learn that some answers lie with our bacteria.
When I say “ecosystem”, most of us will conjure an image of the Great Barrier Reef, the African savannah, or some other amazing macroscopic slice of our environment. What we rarely appreciate is that every person is an ecosystem unto themselves with a wide variety of diverse environments that vast populations of microbes, the microbiota, call home. Our human cells are outnumbered 10:1 by bacterial cells, which in total may weigh 4-6 pounds- the approximate weight of the human brain. The overwhelming majority of these bacteria are our symbionts where we benefit them and they benefit us. In the last 5-10 years, science has begun to understand just how much they benefit us and that having the right numbers, of the right kind of bacteria, at the right times in our lives, can have a tremendous impact on our health. However, we are far from understanding how the symbiotic relationship that we have with our bacteria works, and precisely how their presence or absence contributes to optimal health or disease. Those answers will be transformative to human health.
The human microbiota contains thousands of different species of bacteria, the majority of which are difficult or impossible to manipulate in the lab. This makes understanding the underlying interactions between our cells and our bacteria an intractable problem. The fruit fly, on the other hand, has a very accessible and experimentally friendly microbiota and anatomy, and has proven over decades of research to be a remarkable model for human biology in the areas of immunity, development, and animal behavior, for example.
We are using Drosophila as our model to dissect the gut-microbiota-brain connection: the ways in which the gut microbiota controls brain function and animal behavior. We have assembled a multi-disciplinary team at Carnegie Mellon including Luisa Hiller (microbiologist, Biological Sciences), Carleton Kingsford (computational biologist, Lane Center for Computational Biology), Jonathan Minden (biochemist, Biological Sciences), and Aaron Mitchell (microbiologist, Biological Sciences) as well as a growing number of collaborators outside of the university, to tackle this problem. With transcriptomics and proteomics, we are learning which genes and proteins in the fly are susceptible to manipulation by the bacterial microbiota, and we are discovering how these changes affect the physiology and behavior of the fly in unexpected ways.
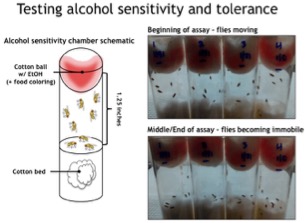 Figure 2: We have found that the microbiota alters Drosophila’s sensitivity to and tolerance of alcohol. In this simple assay, flies with a normal microbiota, or an experimentally altered microbiota, are exposed to ethanol to determine the time to sedation. Like humans, flies develop tolerance to alcohol after repeated exposures and can develop addicitive like behaviors surrounding the acquisition of alcohol. We are investigating how the microbiota may be modulating these responses and behaviors in Drosophila. Interestingly, data in the literature suggest that at least a subset of people with alcohol abuse disorders also have dysbiosis, or alterations to the normal composition of their microbiota, suggesting that alteration of the microbiota could be a risk factor in alcohol abuse. Understanding the biological connections between the microbiota and alcohol use and abuse has the potential to help identify people at risk for developing alcohol use disorders, and mitigate the risk of alcoholism.
Figure 2: We have found that the microbiota alters Drosophila’s sensitivity to and tolerance of alcohol. In this simple assay, flies with a normal microbiota, or an experimentally altered microbiota, are exposed to ethanol to determine the time to sedation. Like humans, flies develop tolerance to alcohol after repeated exposures and can develop addicitive like behaviors surrounding the acquisition of alcohol. We are investigating how the microbiota may be modulating these responses and behaviors in Drosophila. Interestingly, data in the literature suggest that at least a subset of people with alcohol abuse disorders also have dysbiosis, or alterations to the normal composition of their microbiota, suggesting that alteration of the microbiota could be a risk factor in alcohol abuse. Understanding the biological connections between the microbiota and alcohol use and abuse has the potential to help identify people at risk for developing alcohol use disorders, and mitigate the risk of alcoholism.
Notes:
3 ”Missing Microbes: How the Overuse of Antibiotics is Fueling our Modern Plagues” by Martin J. Blaser, M.D. (2014)
