Research
Developmental Regulatory Genomics
The organization, regulation, and function of developmental gene regulatory networks
Development is encoded in the genome. For many years, the genetic basis of development was studied primarily at the level of single genes; i.e., by identifying genes with developmental functions through the use of genetic screens, targeted gene knockouts, and other approaches. More recently, gene regulatory networks (GRNs) have proven to be powerful conceptual and experimental tools for analyzing the genetic control of developmental processes. These elaborate and dynamic networks drive programs of differential gene transcription in embryonic cells. They consist of regulatory genes; i.e, genes that encode transcription factors (TFs), and the cis-regulatory modules (CRMs) to which these TFs bind. GRNs are often depicted using circuit diagrams that indicate interactions among genes in the network. GRN analysis has been driven by many important technical advances, including next-generation DNA sequencing, new approaches for interfering with gene function, and genome-wide approaches for quantifying gene expression, mapping active promoters and enhancers, and identifying transcription factor binding sites.
Developmental GRNs are elaborated in a progressive fashion within specific embryonic lineages or territories. Their deployment can be viewed as layered; cell specification is driven by interactions among early regulatory genes, which leads to the engagement of additional layers of regulatory genes, and finally to the activation of various non-regulatory genes (“differentiation” or “effector” genes) that represent the output of the network. The functions of these terminal genes are often poorly understood, but some encode proteins that play a direct role in driving key morphogenetic processes. Understanding the cellular functions and regulatory control of key effector genes is essential in understanding how anatomy is encoded in the genome.
One major goal of our current work is to gain a detailed understanding of GRN structure and evolution, using as a model a network deployed specifically in the skeleton-forming primary mesenchyme cells (PMCs). Currently, the PMC GRN is one of the most comprehensive in any developing organism and this network has undergone remarkable modifications during echinoderm evolution. Our recent work has exploited new, genome-wide methods for a) chromatin accessibility profiling (ATAC-seq and DNase-seq), b) mapping of transcription factor binding sites (ChIP-seq), and c) identification of enhancer-associated RNAs (eRNAs) to facilitate high-throuhput discovery of CRMs. Individual transcription factor binding sites in these CRMs are now being identified through mutational analysis of reporter genes expressed in transgenic embryos.
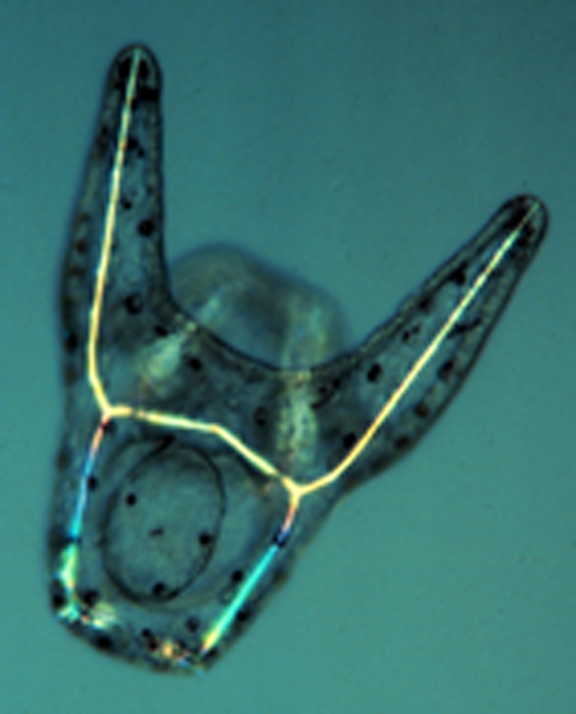
A living, late-stage embryo (early pluteus larva). The formation of the various cell types of the embryo is driven by distinct gene regulatory networks (GRNs) in different cell lineages.
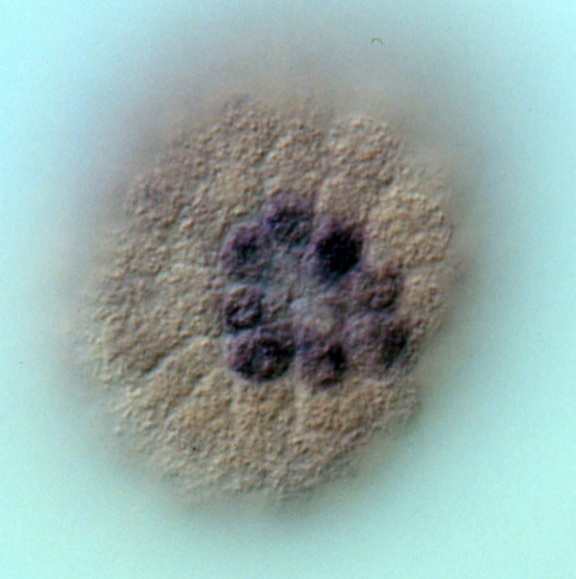
Whole mount in situ hybridization (WMISH) showing the expression of alx1 mRNA in the eight descendants of the large micromeres (purple cells). Alx1 is a lineage-specific transcription factor that regulates many skeletogenic genes.
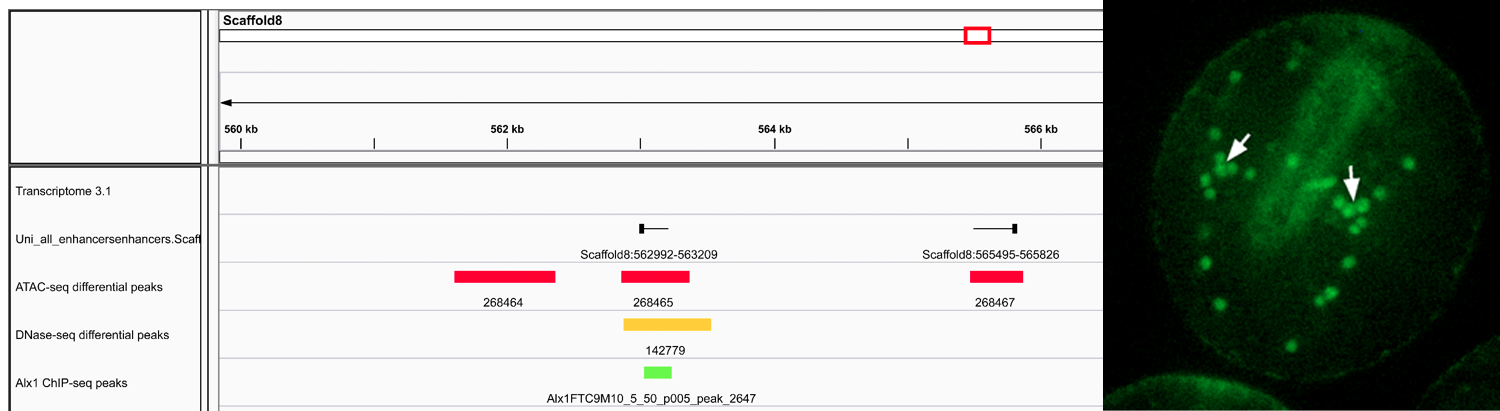
Left- : IGV screenshot showing correspondence between various chromatin features used for CRM discovery. “Differential peaks” indicate regions of chromatin selectively open in PMCs relative to non-PMCs, as determined by ATAC-seq or DNase-seq. “Alx1 ChIP-seq peaks” indicate binding sites for Alx1, a key transcription factor expressed specifically by PMCs. “Uni_all_enhancers” indicates eRNAs. The region of chromatin shown is directly upstream of the Sp-kirrelL gene, which encodes a cell adhesion protein required for PMC-PMC fusion. Data are from the mesenchyme blastula stage. Right- CRMs identified by ATAC-seq drive reporter gene expression selectively in PMCs. Peak #84755 was cloned into the expression plasmid EpGFPII and linearized plasmids were injected into S. purpuratus eggs. 72 hrs after fertilization (prism stage) GFP expression is restricted to PMCs (arrows).
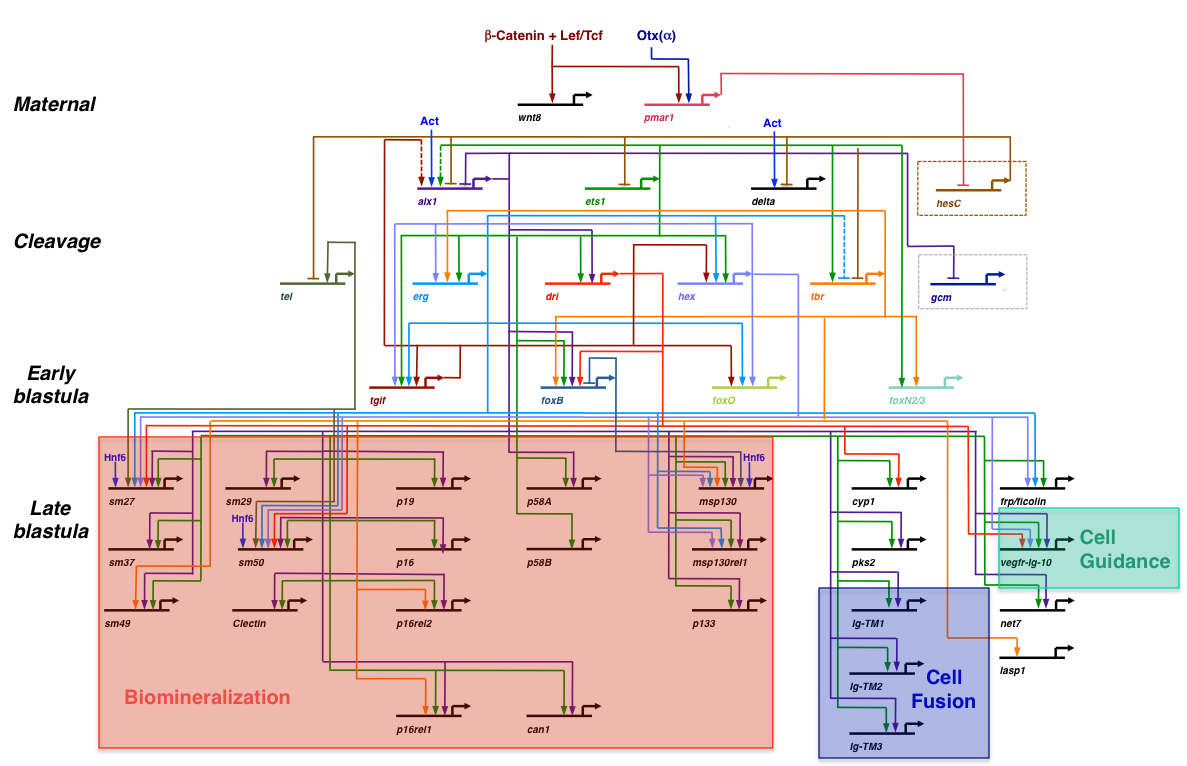
A simplified view of the skeletogenic gene regulatory network (GRN) (modified after Rafiq et al., 2012). This GRN is deployed specifically in the large micromere-PMC lineage. Only a small fraction of the known effector genes are shown here (see Rafiq et al., 2014 for further details).
Linking GRNs to Morphogenesis
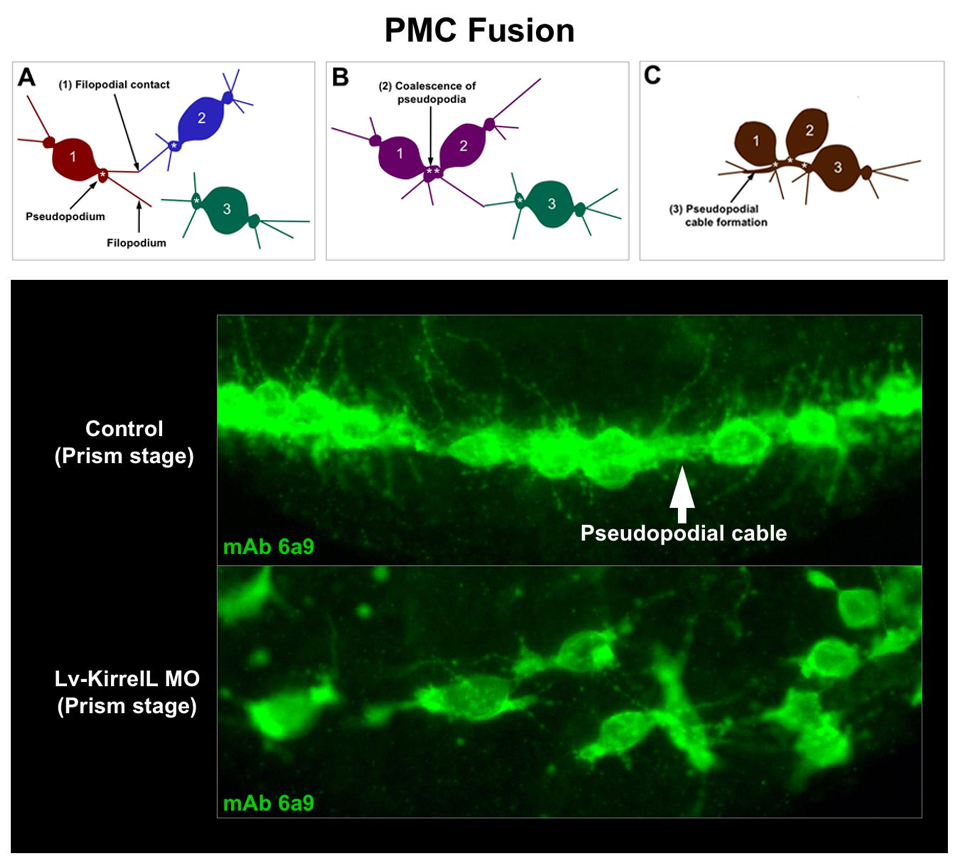
KirrelL, a novel Ig-domain cell adhesion protein, is required for PMC fusion. Upper panel: Diagram of PMC fusion, a process that results in an extensive syncytial PMC network. Lower panel: In the absence of KirrelL function (Lv-KirrelL MO), contacts between PMC filopodia fail to result in cell-cell fusion and the PMC syncytium does not form. The kirrelL gene is expressed specifically by PMCs and is regulated positively by ets1 and alx1, two key regulatory genes in the PMC GRN. CRMs that drive kirrelL expression have been isolated and are being dissected experimentally.
Relevant Papers
Khor JM, Ettensohn CA. An optimized Tet-On system for conditional control of gene expression in sea urchins. Development. 2023 Jan 1;150(1):dev201373. doi: 10.1242/dev.201373. Epub 2023 Jan 6. PMID: 36607745; PMCID: PMC10108607. PDF
Ettensohn CA, Guerrero-Santoro J, Khor JM. Lessons from a transcription factor: Alx1 provides insights into gene regulatory networks, cellular reprogramming, and cell type evolution. Curr Top Dev Biol. 2022;146:113-148. doi: 10.1016/bs.ctdb.2021.10.005. Epub 2021 Dec 10. PMID: 35152981. PDF
Khor JM, Ettensohn CA. Architecture and evolution of the cis-regulatory system of the echinoderm kirrelL gene. Elife. 2022 Feb 25;11:e72834. doi: 10.7554/eLife.72834. PMID: 35212624; PMCID: PMC8903837. PDF
Arshinoff BI, Cary GA, Karimi K, Foley S, Agalakov S, Delgado F, Lotay VS, Ku CJ, Pells TJ, Beatman TR, Kim E, Cameron RA, Vize PD, Telmer CA, Croce JC, Ettensohn CA, Hinman VF. Echinobase: leveraging an extant model organism database to build a knowledgebase supporting research on the genomics and biology of echinoderms. Nucleic Acids Res. 2022 Jan 7;50(D1):D970-D979. doi: 10.1093/nar/gkab1005. PMID: 34791383; PMCID: PMC8728261. PDF
Khor JM, Guerrero-Santoro J, Douglas W, Ettensohn CA. Global patterns of enhancer activity during sea urchin embryogenesis assessed by eRNA profiling. Genome Res. 2021 Jul 30:gr.275684.121. doi: 10.1101/gr.275684.121. Epub ahead of print. PMID: 34330790. PDF
Guerrero-Santoro J, Khor JM, Açıkbaş AH, Jaynes JB, Ettensohn CA. Analysis of the DNA-binding properties of Alx1, an evolutionarily conserved regulator of skeletogenesis in echinoderms. J Biol Chem. 2021 Jul;297(1):100901. doi: 10.1016/j.jbc.2021.100901. Epub 2021 Jun 19. PMID: 34157281; PMCID: PMC8319359. PDF
Bardhan A, Deiters A, Ettensohn CA. Conditional gene knockdowns in sea urchins using caged morpholinos. Dev Biol. 2021 Jul;475:21-29. doi: 10.1016/j.ydbio.2021.02.014. Epub 2021 Mar 5. PMID: 33684434. PDF
Ettensohn CA. The gene regulatory control of sea urchin gastrulation. Mech Dev. 2020 Jun;162:103599. doi: 10.1016/j.mod.2020.103599. Epub 2020 Feb 28. PMID: 32119908. PDF
Khor JM, Ettensohn CA. Transcription factors of the Alx family: evolutionarily conserved regulators of deuterostome skeletogenesis. Front Genet. 2020 Nov 23;11:569314. doi: 10.3389/fgene.2020.569314. PMID: 33329706; PMCID: PMC7719703. PDF
Khor JM, Guerrero-Santoro J, Ettensohn CA. (2019) Genome-wide identification of binding sites and gene targets of Alx1, a pivotal regulator of echinoderm skeletogenesis. Development 146: pii: dev180653. PDF
Shashikant T, Ettensohn CA. (2019) Genome-wide analysis of chromatin accessibility using ATAC-seq. Methods Cell Biol. 151:219-235. PDF
Buckley KM, Ettensohn CA. (2019) Techniques for analyzing gene expression using BAC-based reporter constructs. Methods Cell Biol. 151:197-218. PDF
Shashikant T, Khor JM, Ettensohn CA. (2018) From genome to anatomy: The architecture and evolution of the skeletogenic gene regulatory network of sea urchins and other echinoderms. Genesis 56:e23253. PDF
Sun Z, Ettensohn CA. TGF-β sensu stricto signaling regulates skeletal morphogenesis in the sea urchin embryo. Dev Biol. 2017 Jan 15;421(2):149-160. PDF
Ettensohn CA, Dey D. KirrelL, a member of the Ig-domain superfamily of adhesion proteins, is essential for fusion of primary mesenchyme cells in the sea urchin embryo. Dev Biol. 2017 Jan 15;421(2):258-270. PDF
Ettensohn, C. A. (2014). Horizontal transfer of the msp130 gene supported the evolution of metazoan biomineralization. Evol. Dev 16: 139-148. PDF
Rafiq, K., Shashikant, T., McManus, C. J., and Ettensohn, C. A. (2014). Genome-wide analysis of the skeletogenic gene regulatory network of sea urchins. Development 141: 950-961. PDF
Adomako-Ankomah, A. and Ettensohn, C. A. (2014). Growth factors and early mesoderm morphogenesis: insights from the sea urchin embryo. Genesis 52: 158-172. PDF
Adomako-Ankomah, A. and Ettensohn, C. A. (2013). Growth factor-mediated mesodermal cell guidance and skeletogenesis during sea urchin gastrulation. Development 140: 4214-4225. PDF
Ettensohn, C. A. (2013). Encoding anatomy: developmental gene regulatory networks and morphogenesis. Genesis 51: 383-409. PDF
Rafiq, K., Cheers, M., and Ettensohn, C. A. (2012). The genomic regulatory control of skeletal morphogenesis in the sea urchin. Development 139: 579-590. PDF
Flynn, C. J., Sharma, T., Ruffins, S. W., Guerra, S. L., Crowley, J. C., and Ettensohn, C. A. (2011). High-resolution, three-dimensional mapping of gene expression using GeneExpressMap (GEM). Dev. Biol 357: 532-540. PDF
Adomako-Ankomah, A. and Ettensohn, C. A. (2011). P58-A and P58-B: novel proteins that mediate skeletogenesis in the sea urchin embryo. Dev. Biol. 353: 81- 93. PDF
Sharma, T. and Ettensohn, C. A. (2010). Activation of the skeletogenic gene regulatory network in the early sea urchin embryo. Development 137: 1149-1157. PDF
Ettensohn, C. A. (2009). Lessons from a gene regulatory network: echinoderm skeletogenesis provides insights into evolution, plasticity, and morphogenesis. Development 136: 11-21. PDF
Livingston, B. T., Killian, C. E., Wilt, F., Cameron, A., Landrum, M. J., Ermolaeva, O., Sapojnikov, V., Maglott, D. R., Buchanan, A. M., and Ettensohn, C. A. (2006). A genome-wide analysis of biomineralization-related proteins in the sea urchin, Strongylocentrotus purpuratus. Dev. Biol. 300: 335-348. PDF
Sea Urchin Genome Sequencing Consortium (2006). The genome of the sea urchin Strongylocentrotus purpuratus. Science 341: 941-952. PDF